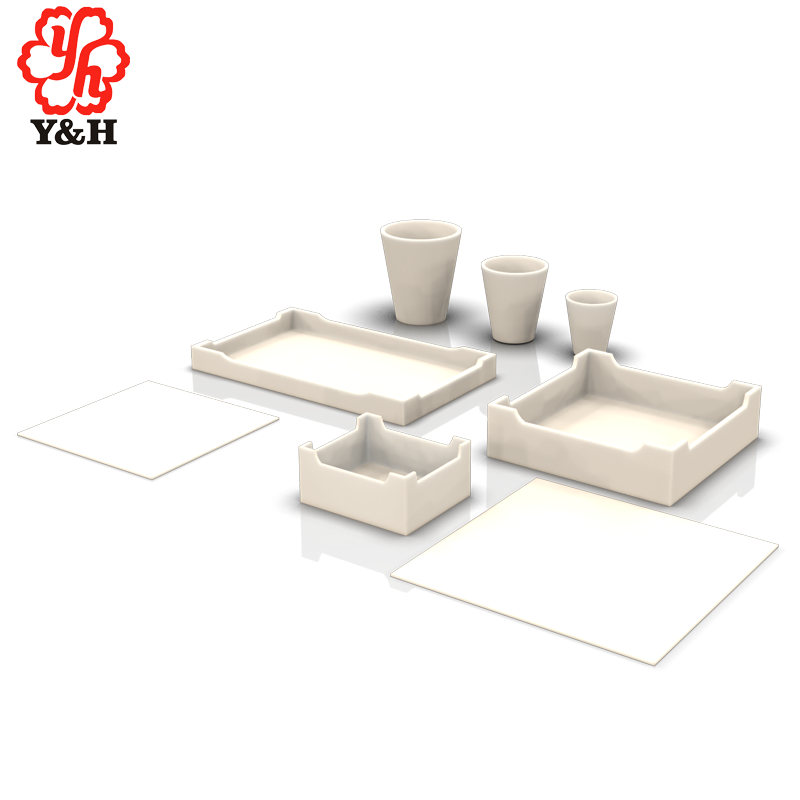
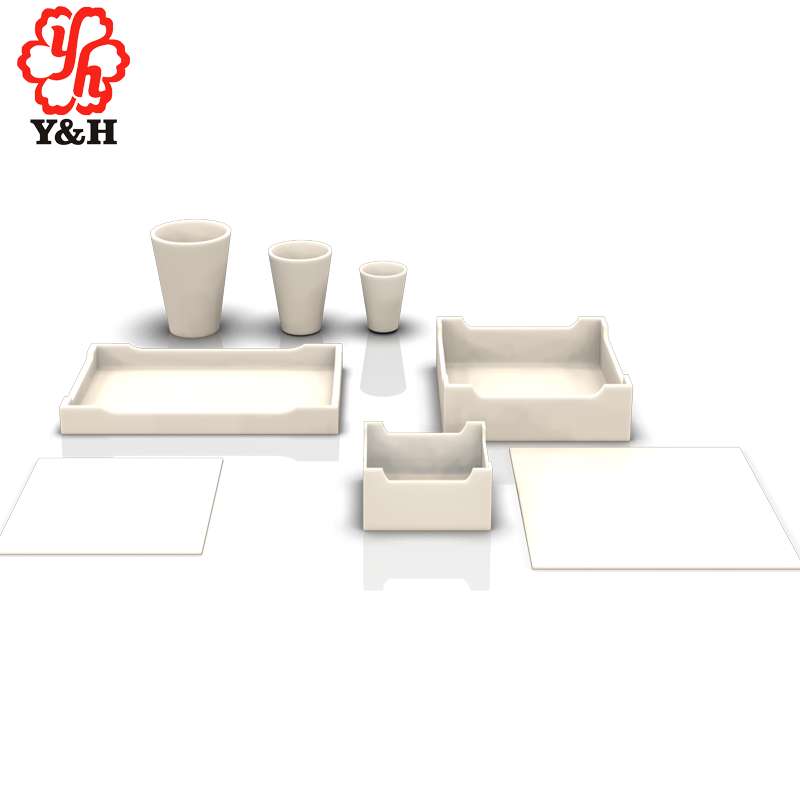
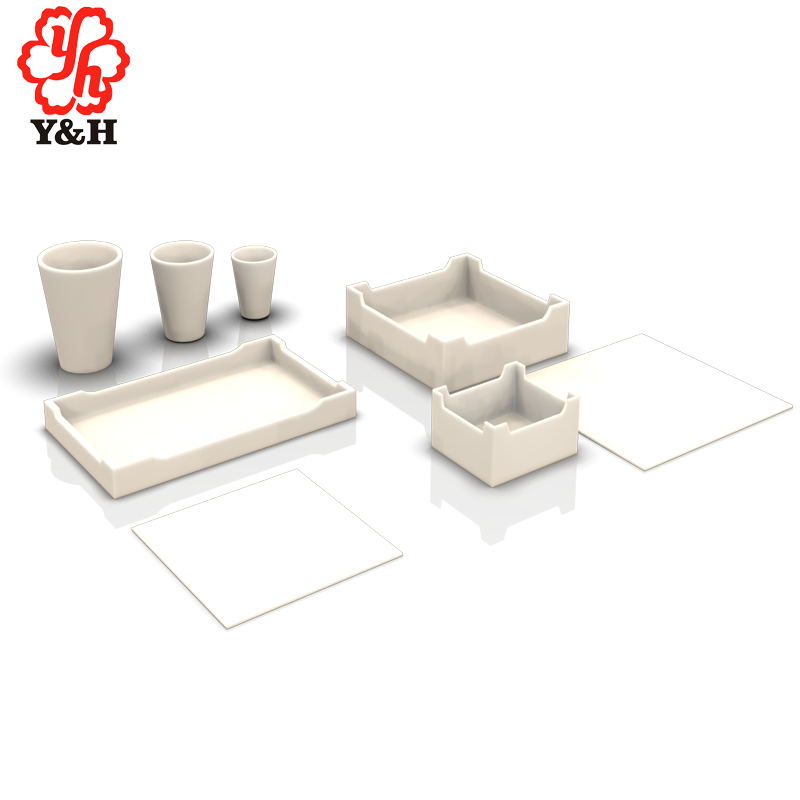
Zirconia Crucibles
Zirconia crucible with a purity of 99.9%, density of 6.00, and a maximum operating temperature of 2200 degrees Celsius, primarily used for melting precious metals. It exhibits excellent heat stability and is mainly employed for melting rare precious metals. Zirconia crucibles have good high-temperature stability and are ideal refractory products for experimental purposes.
Zirconium dioxide has a melting point higher than zirconium, reaching 2700°C, making it one of the most refractory materials in nature. It has poor thermal conductivity but strong electrical conductivity and erosion resistance. Even when heated to over 1900 degrees Celsius, it does not react with molten metals such as aluminum, iron, nickel, platinum, silicates, or acidic furnace slags. Therefore, it is used to manufacture crucibles for melting precious metals, refractory tubes, heat-resistant glass, and enamel. Adding zirconium dioxide to enamel and glass can enhance their resistance to acid and alkali corrosion. High-temperature furnaces lined with zirconium dioxide expand minimally when heated and are less affected by temperature changes, reducing the risk of cracking due to thermal expansion and contraction, thereby significantly extending the furnace's lifespan. When zirconium dioxide is used as a refractory material with 5% calcium oxide as a stabilizer, its heat resistance temperature is 500 degrees higher than alumina, and its insulating capacity is tripled compared to before the addition. Adding white zirconium dioxide to ceramics makes them whiter, brighter, and more heat-resistant, with increased strength. This type of ceramic is used to manufacture high-temperature insulation bottles, providing strong insulation capability and minimal expansion coefficient.
Here are the typical parameters and specifications for zirconium dioxide (ZrO2):
Melting Point: Approximately 2700°C
Thermal Conductivity: Relatively low compared to metals, but varies depending on the phase (cubic or tetragonal) and temperature.
Electrical Conductivity: Zirconium dioxide exhibits good electrical conductivity.
Chemical Stability: Highly resistant to corrosion by acids, bases, and molten metals.
Expansion Coefficient: Low, minimizing the risk of cracking due to thermal expansion.
Insulating Properties: High insulating capacity, especially when stabilized with additives like calcium oxide (CaO).
Applications: Used in high-temperature applications such as crucibles for melting precious metals, refractory linings in furnaces, heat-resistant glass, and enamel.
These parameters make zirconium dioxide a versatile material in industries requiring high-temperature stability, resistance to chemical corrosion, and strong insulating properties.